Incorporating Structural and Recognition Elements in the Structure of Abiotic Phosphates
Research Directions
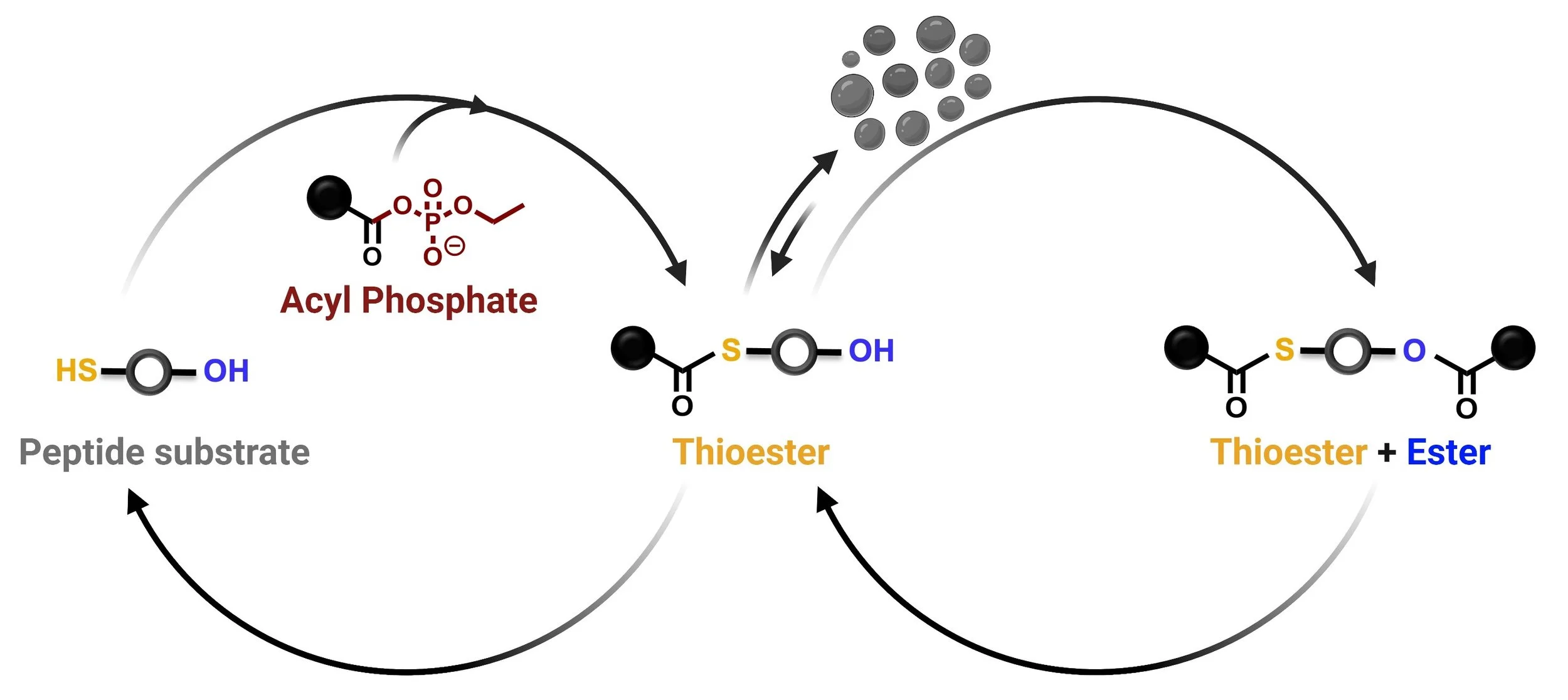
Abiotic Oligomerization using Amino Acyl Phosphates
Our research investigates non-enzymatic peptide bond formation via aminoacyl phosphate esters. By studying these processes in abiotic conditions, we aim to mimic mechanisms relevant to prebiotic systems chemistry. We focus on how environmental factors influence oligomerization and self-assembly, shedding light on novel synthetic pathways. We aim to understand how phase behavior and dynamic systems can control peptide sequence selection and assembly.
Reaction Networks using ‘‘Structured Fuels’’
Inspired by biological systems like ATP and GTP, our research explores how amino acyl phosphates drive dynamic reaction networks with adaptive behavior. By training materials to respond to energy inputs and competitive pathways, we investigate how structural and recognition elements enable programmable functions in chemical systems. These systems navigate complex energy landscapes, altering their structure in real-time and offering insights into ‘‘structured fuels’’.
Dynamic Re-Phosphorylation
We aim to unravel the mechanisms that allow dynamic re-phosphorylation to drive transient non-equilibrium states in peptide systems. This process facilitates reversible phosphorylation, controlling reaction networks that lead to the assembly and disassembly of abiotic materials. Through this approach, we gain insights into early biochemical processes while developing dynamic materials with potential applications in catalysis and soft peptide materials.